
wildpixel
On Thursday, September 14, Anavex Life Sciences (NASDAQ:AVXL), a clinical-stage biopharmaceutical company which develops small molecule drugs for neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease, Rett syndrome etc. announced additional data from their phase 2b/3 Alzheimer’s Disease trial.
Previously (on December 1, 2022), the company announced the topline data from this trial, which I covered in this SA article.
Before I dive into the data, let’s first take a look at the trial protocol or design (as presented in the company’s latest presentation, slide 16).
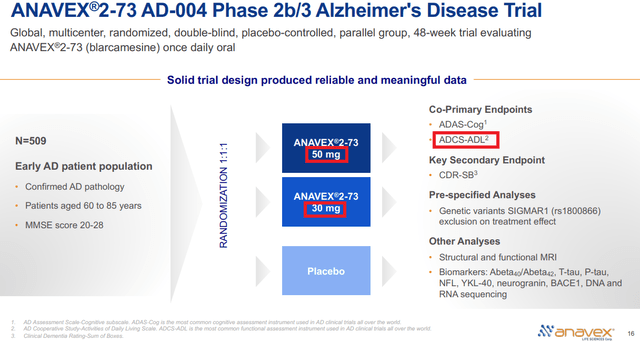
Anavex May 2023 Presentation
(Red boxes added)
As seen above, in this trial there are two “co-primary endpoints” which are ADAS-Cog and ADCS-ADL and one “key secondary endpoint” which is CDR-SB.
Another thing to note here is that the trial is randomized (1:1:1) into three groups: placebo and two treatments groups of different doses (30mg and 50mg).
In the remainder of this article, I’d like to list and compare the results reported in the two PRs (December 1, 2022 vs. September 14, 2023).
Headlines & Sub-headlines
The table below shows the headlines and sub-headlines of Anavex’s two PRs:
| Headlines |
 Anavex PR (Dec 1, 2022) |
 Anavex PR (Sep 14, 2023) |
| Sub-headlines |
 Anavex PR (Dec 1, 2022) |
Anavex PR (Sep 14, 2023) |
An observation here: It seems to me that the September 14’s PR is more modest in its claims about the results.
Next we’ll turn to safety data.
Safety Data
On Safety, this is what the company reported on December 1, 2022: “ANAVEX®2-73 (blarcamesine) was generally safe and well tolerated. The incidence of treatment emergent adverse events (TEAEs) was similar in the active and placebo arms with dizziness being the most common TEAE. TEAEs ≥7.5% threshold were predominantly mild or moderate. No clinically significant changes in vital signs, laboratory values and ECG parameters in active and placebo arms were observed. Safety findings in the study were consistent with the known safety profile of ANAVEX®2-73.” (bold emphasis added) |
On safety, this is what the company reported on September 14, 2023: “In the respective safety population, common treatment-emergent adverse events included dizziness, which was transient and mostly mild to moderate in severity, and occurred in 120 participants (35.8%) during titration and in 76 participants (25.2%) during maintenance with blarcamesine and 10 (6.0%) during titration and 9 (5.6%) during maintenance with placebo.” |
The latest update has more details on safety, which is summarized in the table below.
TEAEs During titration | TEAEs during maintenance | |
| Treatment groups | 35.8% | 25.2% |
| Placebo | 6% | 5.6% |
(Compiled by author)
As the company did not comment on the updated safety data, I don’t know if they still consider the incidence of TEAEs in treatment groups and placebo to be “similar” as previously announced, though they are numerically much bigger in the treatment groups.
Biomarker data
There was no biomarker data reported previously. So it’s all in the September 14 PR:
…validated biomarkers of amyloid beta pathology, plasma Aβ42/40 ratio increased significantly (P = 0.048), demonstrating strong anti-amyloid effects of blarcamesine in Alzheimer’s disease patients, while MRI revealed significant reduction in brain volume loss, including whole brain (P = 0.0005), comparing treatment to placebo.
Regarding this new biomarker data, the company stated in the PR:
This (blarcamesine (ANAVEX®2-73)) is among the first drugs to prospectively demonstrate efficacy on biomarkers of neurodegeneration.
Next, let’s turn to the efficacy data.
Efficacy data: “The trial is successful in meeting the co-primary endpoints if…, or if…”
For anyone who is surprised by the title of this paragraph or finds it odd, here is the entire paragraph in the September 14 PR, regarding the efficacy data:
All prespecified clinical endpoints were analyzed using a mixed model for repeated measures (MMRM). The MMRM analysis method is the convention used for regulatory filings and discussions with regulatory authorities are in preparation.
The trial is successful in meeting the co-primary endpoints if the significance of each endpoint is P < 0.05, or if the significance of only one co-primary endpoint is P < 0.025. If only one primary endpoint is significant at an α level of 0.025, then the secondary endpoint will be evaluated at the same level of 0.025. The trial was successful, since the differences in the least-squares mean (LSM) change from baseline to 48 weeks between the blarcamesine and placebo groups were −1.783 (95% CI, −3.314 to −0.251); (P = 0.0226) for ADAS-Cog13, and −0.456 (95% CI, −0.831 to −0.080); (P = 0.0175) for CDR-SB in patients with early Alzheimer’s disease.
Or in summary, the results here, and what was reported on December 1, 2022:
*Note: In Dec 1 PR, the company reported the differences in mean score change from baseline to 48 weeks between treatment groups and placebo vs. the differences in “the least-squares mean (LSM) change” of the same period and between placebo and treatment groups.
One probably needs to have an advanced degree in medical statistics to understand how the new post hoc analysis transforms the ADAS-Cog13 and CDR-SB data from trial failure to trial success (i.e. to be under the critical p <0.025 limit, according to this new criteria of success disclosed/defined in the September 14 PR).
For those who don’t, yours truly included, it’s sufficient to know that according to Anavex, their p2b/3 AD trial was successful, because it has shown statistically-significant efficacy data in one pre-specified primary endpoint, namely ADAS-Cog 13, and in the secondary endpoint, CDR-SB, and the p values in these two measurements have improved (from the topline data, >0.025, disclosed on December 1, 2022) to become <0.025.
Discussion
I have a few questions that I want to raise here.
1. Where is (per protocol, non-derived) ADCS-ADL data?
In my previous article, I commented on the fact that Anavex did not disclose one of the two co-primary endpoint’s, namely ADCS-ADL, per protocol, non-derived topline data, even though the December 2022 PR claimed the derived ADCS-ADL results were also statistically-significant.
This time, there’s no ADCS-ADL data at all, per protocol, or post-hoc.
2. Why still the pooled data?
Previously, the company explained the use of pooled data (combining 30mg and 50mg groups together) was because those separate data points had not been “run through.”
Almost nine months later, is this still the case?
How does the FDA approve a drug’s go-to-market dose, based on pooled results from two arms?
Is the reason for reporting the pooled data because either one or both treatment arms have no statistically-significant data on their own?
3. No update on the pre-specified group’s “more efficacious” data
Previously, Anavex reported:
…a pre-specified analysis of patients without SIGMAR1 gene mutation provides further confidence of the robustness of the SIGMAR1 activation in the treatment of neurodegenerative diseases. Approximately 80% of the total worldwide population lack a SIGMAR1 gene mutation.(5) ANAVEX®2-73 was more efficacious in this pre-specified population. This effect is consistent with prior clinical trials of ANAVEX®2-73.(6)
(Bold emphasis added)
At that time, the company did not disclose any actual data to support the “more efficacious” claim. Nor has the company done so in the recent update.
4. Has Anavex met with any regulator yet?
In the December 2022 PR, Anavex said:
“Next step, in light of this data, is meeting with regulatory authorities to discuss this data in the context of ongoing development with an aim to bring this therapy to patients in Europe, Asia-Pacific, and the U.S.”
And in the latest PR, they said:
“discussions with regulatory authorities are in preparation.”
Reading these two statements, it is unclear whether or not any meeting or discussion with the regulators has already taken place, or if the company is “in preparation” to meet and discuss with the regulators.
Financials
According to the latest 10Q, the company has a cash runway of $154.8M, as of June 30, 2023.
The quarterly net loss is ~$12.5M, which would imply a 3-year cash runway, provided that the burn rate stays relatively stable as the recent quarters.
Conclusion
Between my last and current article on Anavex, I have sold my AVXL stake and exited the AD space entirely as a biotech investor.
For reasons discussed in my previous and current article, I find AVXL’s AD program riddled with problematic inconsistencies.
The risk of loss (p3 failure) here is, in my opinion, very high and therefore as a long-term investment opportunity, it’s probably only suitable for investors who have extremely high risk tolerance, and who know how to size their stake appropriately to reflect that risk.
Thanks for reading! Hope this article is clear and helpful for your own research into this company.



